 Copyright © Michael Richmond.
This work is licensed under a Creative Commons License.
Copyright © Michael Richmond.
This work is licensed under a Creative Commons License.
Way back around the turn of the twentieth century, physicists were starting to peer into the sub-atomic realm. Their instruments were finally growing sophisticated enough to break into atoms and detect individual particles.
To simplify greatly, scientists determined that atoms were made of smaller particles. The earliest ideas were that these particles came in two varieties:
They figured out quickly that electrons were less massive and easier to manipulate than protons, since the positive charges were clumped together near the center of atoms in a dense little ball we call the nucleus.
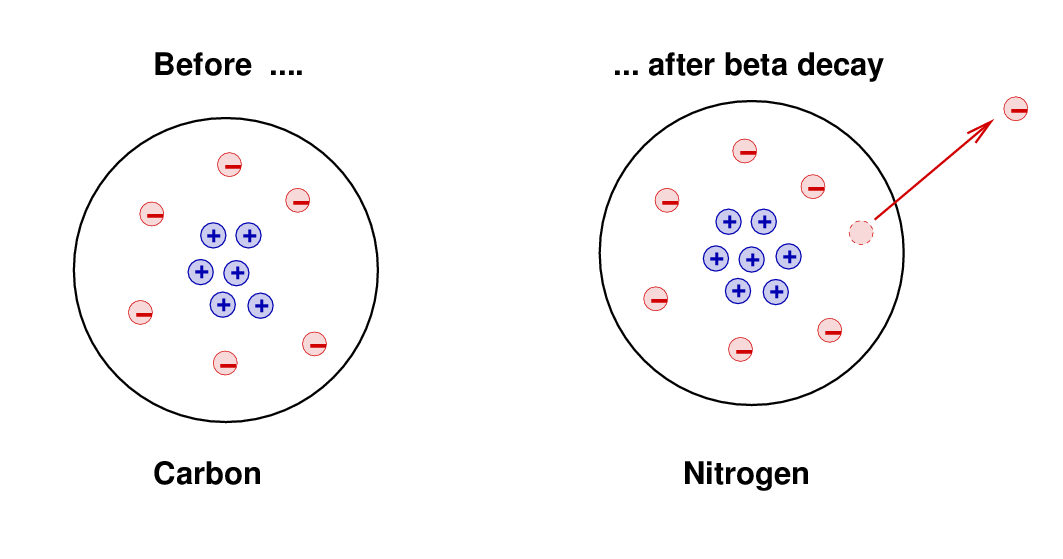
Some atoms were observed to decay spontaneously into new forms -- a process called radioactivity. One type of radioactivity, known as beta decay, involved the creation of a proton and electron together, after which the electron shot away from the atom with a high energy and momentum. For example,

The original atom had 6 protons and 6 electrons, and was electrically neutral. The daughter atom had 7 protons and (briefly) 7 electrons, and so was also electrically neutral. This conservation of electric charge was one of the most stringently tested and proven principles of chemistry and physics, so it was a relief to see that the strange new process of radioactivity obeyed the rule.
However ... there were other properties that seemed NOT to be conserved by this process. For example, the electron shot away from the atom with a high speed, taking energy and momentum with it. The atom recoiled a bit, but careful measurements showed that the total energy of the (daughter-atom-plus-electron) wasn't quite as large as the energy of the original atom; moreover, the momentum of the (daughter-atom-plus-electron) was also not equal to the momentum of the original atom. It appeared that beta decay violated conservation of energy and conservation of momentum. This was bad.
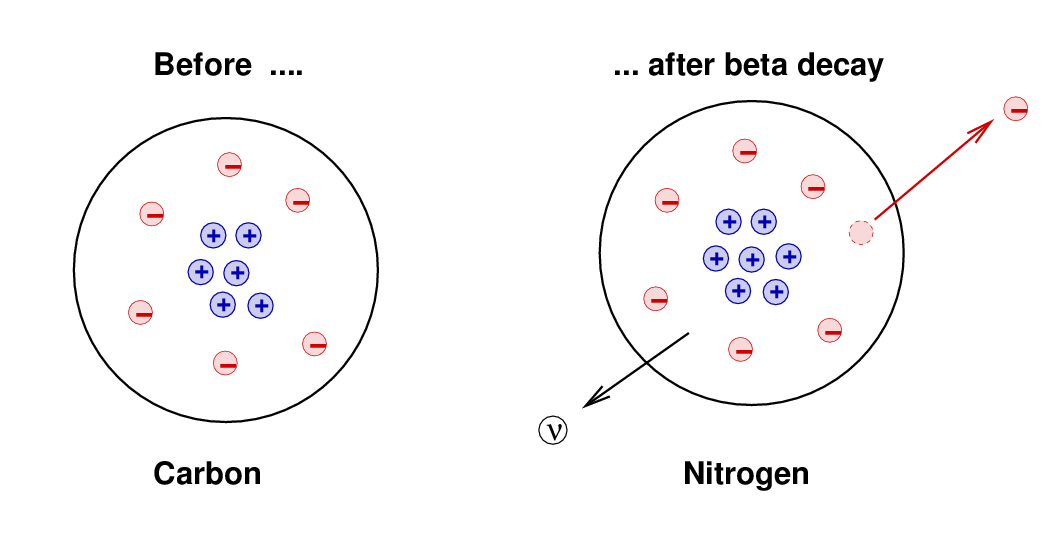
In 1930, Wolfgang Pauli suggested that perhaps a third particle was involved in beta decay. Suppose, he said, that when a proton and electron are created within an atom, a NEUTRAL particle is also created. This neutral particle would carry away some small amount of energy and momentum, just enough to bring the final values into balance with the original ones.
In 1932, a neutral particle was found, and was given the name neutron. Alas, it did not have the right properties to fix the problems with beta decay. Rats.
When physicists realized that the neutron could not explain the missing energy and momentum, they postulated that a different neutral particle must also exist. Since this particle seemed to have a small mass, it was given the name neutrino, an Italian diminuitive meaning "cute little neutral particle." (Well, maybe without the "cute").
After many years of searching, the neutrino was finally detected in 1956 by a team led by Clyde Cowan and Frederick Reines. For this prodigious experimental feat, Reines was awarded a share of the Nobel Prize in Physics in 1995; Cowan, unfortunately, had died in 1974, and was thus ineligible for his share of the Prize.
Why did it take scientists so long to detect this particle? One reason is its lack of charge: particles with electric charge exert large forces on other particles, and so are easy to notice. But it turned out that the neutrino was a very strange particle: it interacted very, very, VERY weakly with matter, much more weakly than any other particle.
How weakly? Well, let's compare it to other items produced by radioactive decay processes. Particles which interact strongly will bump into atoms very soon after entering a chunk of matter, while those which interact weakly will pass through some distance before colliding with an atom.
| Name | also known as | can be stopped by |
| alpha ray | helium nucleus | a sheet of paper, a layer of skin |
| beta ray | electron | several sheets of aluminum foil |
| gamma ray | high energy photon | lead bricks |
These properties show that alpha particles interact strongly with matter, while gamma rays interact relatively weakly.
What about neutrinos?
Well, in order to have a decent chance of blocking a single neutrino, one would have to set up a thick wall of matter. A VERY thick wall. In rough terms, if one placed lead bricks in a row, one would need a line of bricks stretching for about ten light years (or about 1017 meters).
In other words, neutrinos pass through the Earth's atmosphere, the walls of a building, the bodies of humans -- even the entire Earth itself -- and just keep on going. No wonder it took scientists decades to devise a way to detect them.
 Copyright © Michael Richmond.
This work is licensed under a Creative Commons License.
Copyright © Michael Richmond.
This work is licensed under a Creative Commons License.